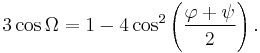310 helix
A 310 helix is a type of secondary structure found (rarely) in proteins.
Structure
The amino acids in a 310-helix are arranged in a right-handed helical structure. Each amino acid corresponds to a 120° turn in the helix (i.e., the helix has three residues per turn), and a translation of 2.0 Å (= 0.2 nm) along the helical axis, and has 10 atoms in the ring formed by making the hydrogen bond. Most importantly, the N-H group of an amino acid forms a hydrogen bond with the C = O group of the amino acid three residues earlier; this repeated i + 3 → i hydrogen bonding defines a 310-helix. Similar structures include the α-helix (i + 4 → i hydrogen bonding) and the π-helix i + 5 → i hydrogen bonding).
310 helix - observed in some proteins, less common than α-helix. The 310 helix is right handed. The 310 helix is frequently found within α-helical segments of proteins including myoglobin (participates in O2 storage in muscle). <source: Principles of Biochemistry 5th edition, David L Nelson, Micheal M Cox)>
Residues in 310-helices typically adopt (φ, ψ) dihedral angles near (−49°, −26°). More generally, they adopt dihedral angles such that the ψ dihedral angle of one residue and the φ dihedral angle of the next residue sum to roughly −75°. For comparison, the sum of the dihedral angles for an α-helix is roughly −105°, whereas that for a π-helix is roughly −125°.
The general formula for the rotation angle Ω per residue of any polypeptide helix with trans isomers is given by the equation
See also
References
- Pauling L, Corey RB and Branson HR. (1951) "The Structure of Proteins: Two Hydrogen-Bonded Helical Configurations of the Polypeptide Chain", Proc. Nat. Acad. Sci. Wash., 37, 205.
|
|||||||||||